
Aperio PeerReview - Streamlined Productivity in Drug Safety Testing
Request a demo from our experts today!
The new viewer is a major leap forward. The additional features enhance the digital slide viewing experience; the auto raster tool is one of my favorites.
--Erio Barale-Thomas, Principal Scientist, Johnson & Johnson, R&D
The pharmaceutical industry is under increasing pressure to streamline workflows and employ lean solutions to reduce the time and cost associated with drug development.
Aperio PeerReview is a web-based, enterprise-level, digital pathology software solution, specifically designed and developed for toxicologic pathologists. By reducing time wasted for unnecessary travel or waiting for slides to be shipped, Aperio PeerReview streamlines the drug safety testing workflow, and facilitates rapid collaboration across multisite biopharmaceutical companies and contract research organizations.
Request a no-obligation demo today from one of our experts
For Research Use Only. Not for use in diagnostic procedures.
 |
The sophisticated, inbuilt communication interface enables integration of Aperio PeerReview with your LIMS for efficient, automated data transfer, eliminating manual data entry and reducing the risk of typographical errors. With options for barcode automation through to data upload, Aperio PeerReview can integrate into your workflow. Unidirectional data flow into Aperio PeerReview software ensures that the integrity of your LIMS data is unaffected by the peer review process and observations. |
 |
Quickly and easily connect to share peer review slides and data with colleagues based across multiple sites within a global organization or with external 3rd party collaborators. The web-enabled software facilitates rapid and secure access to content and colleagues. With installation configurations for single site, multi-site hub & spoke, on premise or cloud deployments, Aperio PeerReview is readily implemented, with minimal IT overhead. |
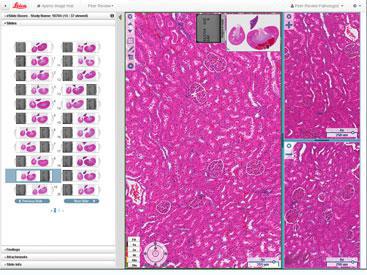 |
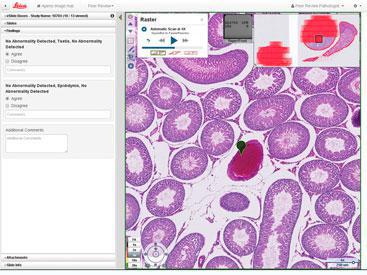
Execute peer review studies quickly and easily with a streamlined workflow designed and developed specifically for the unique needs of toxicologic pathologists. The multi-slide viewer enables pathologists to display control slides and the review slide within the same window, providing easy comparison of morphology, not currently possible with a microscope. Automatic sorting of eSlide Boxes with just a few mouse clicks eliminates the need to physically arrange glass slides, while the integrated data display enables viewing of slides, primary review findings and peer review observations in a single window. |
 |
Reduce time and money spent shipping slides and eliminate unnecessary, expensive travel with instant, remote access to peer review content. With a dashboard overview, users can see all active studies and status, meaning that new peer review studies can be assigned with the touch of a button, based on pathologist workload and capacity. The high-performance software and viewing capabilities facilitates instant, simultaneous review of content for rapid resolution of discordances, enabling faster completion of peer review studies and pathology reports. |
